Russia's Sputnik V vaccine is over 91% efficient at defending towards the coronavirus, based on preliminary trial outcomes. The interim evaluation, printed Tuesday within the journal The Lancet, relies on knowledge from almost 20,000 contributors, based on researchers. The information comes after Russia's fast authorization and rollout prompted skepticism throughout the globe that the vaccine was predominantly a propaganda software for Russian President Vladimir Putin. "Information exhibits #SputnikV is 1 of the three vaccines on the planet with efficacy over 90% and 100% safety from extreme instances," Russia's Twitter account for the vaccine posted. "Different benefits – storage temperature, confirmed security and price – make it a vaccine for all humankind."The examine acknowledged that it solely included instances of symptomatic COVID-19 sufferers and that additional evaluation must be performed on asymptomatic infections. However the outcomes align with interim checks carried out in Russia, the place officers have reached agreements with different nations to mass produce and distribute the vaccine globally.An accompanying commentary on the examine from scientists not related to the analysis stated that "the event of the Sputnik V vaccine has been criticised for unseemly haste, nook chopping, and an absence of transparency.""However the consequence reported right here is obvious and the scientific precept of vaccination is demonstrated, which implies one other vaccine can now be part of the battle to cut back the incidence of COVID-19," British scientists Ian Jones and Polly Roy wrote. [ MAP: The Spread of Coronavirus ]The Russian Direct Funding Fund, the nation's sovereign wealth fund, stated in early January that over 1 million Russians had acquired the vaccine. Some native media shops raised doubts over the quantity and instructed the rollout has been sluggish, based on the Related Press. The 2-dose vaccine has been licensed by over a dozen nations. A spokesperson for the fund instructed the Related Press that greater than 50 nations have submitted purposes for two.4 billion doses.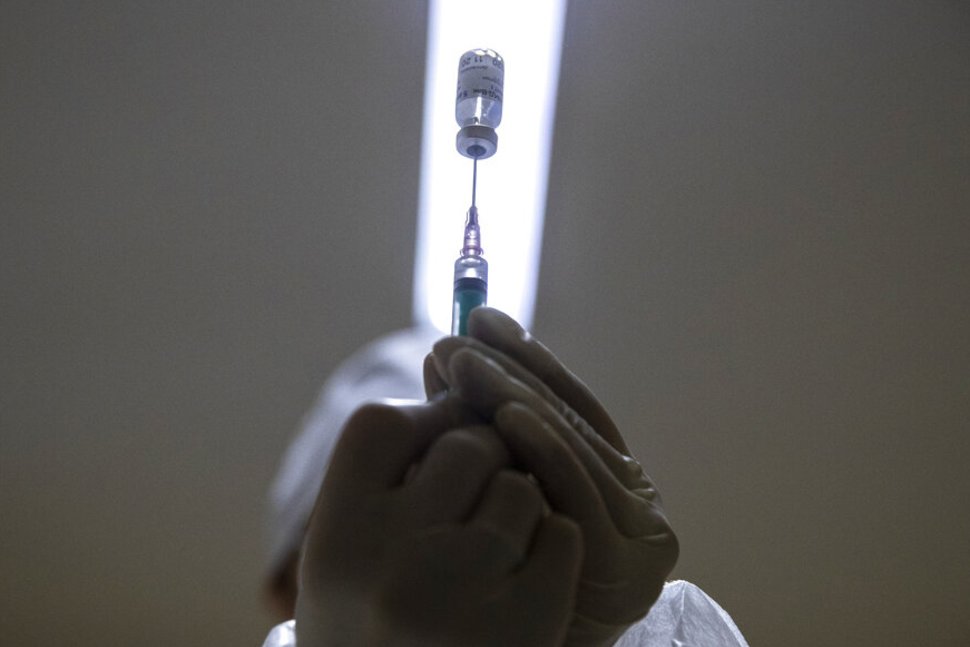
Research Finds Russia’s Sputnik V Vaccine Extremely Efficient in Trial
0
0
SHARES
9
VIEWS
Leave a Reply Cancel reply
BROWSE BY CATEGORIES
BROWSE BY TOPICS
Alexa Lardieri
Associated Press
Best States
Best Stocks
Biden
business
Business News
China
Collections: Business
Collections: Top News
Collections: US
coronavirus
Donald
economy
education
Education Lab
elections
Financial Advisors
foreign policy
health
Healthiest Communities
Healthiest Communities Health News
Health News
infectious diseases
Investing 101
Joe
Lisa Hagen
lung disease
National News
News
Paul D. Shinkman
personal finance
picks
Politics
public health
Russia
Stock Market News
Susan Milligan
Trump
United States
vaccines
Washington Whispers
world
world news
World Report
Archives
- June 2022
- May 2022
- April 2022
- March 2022
- February 2022
- January 2022
- December 2021
- November 2021
- October 2021
- September 2021
- August 2021
- July 2021
- June 2021
- May 2021
- April 2021
- March 2021
- February 2021
- January 2021
- December 2020
- November 2020
- October 2020
- September 2020
- August 2020
- July 2020
- June 2020
- May 2020
Nsapk.com is the driving force behind decisions that largely define the quality of our readers’ and users’ lives.
- Trending
- Comments
- Latest
Biden to Faucet Former Sen. Invoice Nelson to Lead NASA
April 11, 2021
Neera Tanden Withdraws Her Nomination for OMB Director
August 17, 2021
Copyright @ 2021 Nsapk.com. All rights reserved.









